The individual who appears is for illustrative purposes. The person depicted is a model and not a real patient.
Efficiency, contrast utilization, and safety MAXimized
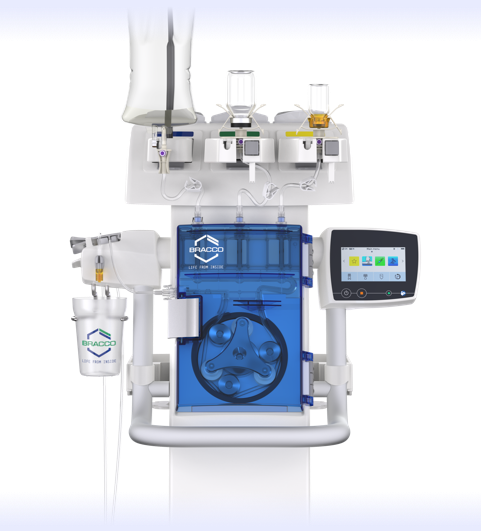
The Bracco Max 3™, a Rapid Exchange and Syringeless MR Injector System
MAXimize Efficiency¹
- Simplifies your workflow with a rapid patient changeover
- Easy-Click-Cassette -flex eliminates syringes for efficient throughout
- 18-hour lithium-ion battery provides cable free footprint and extended performance in MRI suite
MAXimize Savings & Sustainability¹
- Reduce contrast waste with the CM-Loop function
- Optimize your contrast delivery with CM-Select function
- May help meet initiatives in reduction of medical waste
MAXimize Safety & Hygiene¹
- Enjoy unique, automatic air detection technology and particle filtration
- Provides a closed system that minimizes manual contact
- Integrated SafeConnect with Touch Protection helps you maintain aseptic technique
MAXimize Operations¹
- Quick and easy to operate
- Intuitive user interface with step-by-step instructions
- Built in guidance and real-time prompts ensure confidence and consistency
- Dedicated WIFI connection provides direct wireless communication
Experience the Max 3 System below
Hover over icons to see the details


Direct Connection
• Eliminates syringes
• Enhances workflow efficiency within a closed system
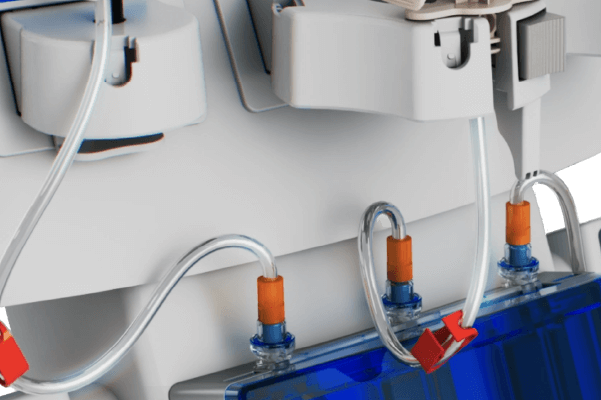
Air Detection Technology
• Automatically stops injection when >1 mL of air is detected in patient line
• Integrated air filters within media spikes


SafeConnect
• Pressure sensor
• Touch protection on consumables to maintain aseptic technique


Drip Cup
• Promotes clean utilization
• Enhances hygiene initiatives
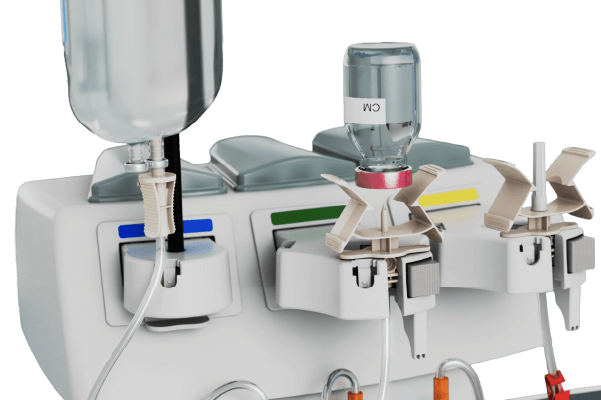
Media Connections
• 1 Saline and 2 media connection points
• Allows for multi-dose/multi-patient workflow
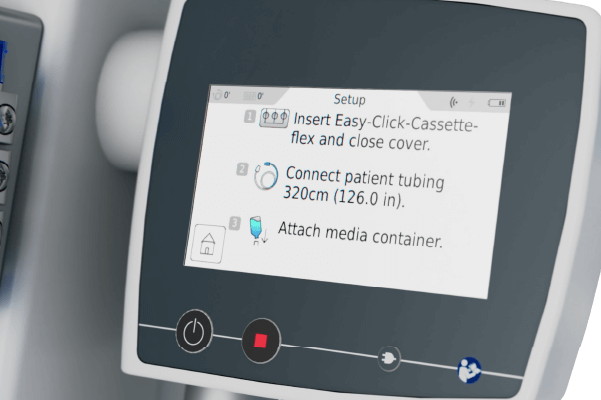
Injector Control Unit (ICU)
• Intuitive operation with touch display
• Provides status updates and step‐by‐step instructions
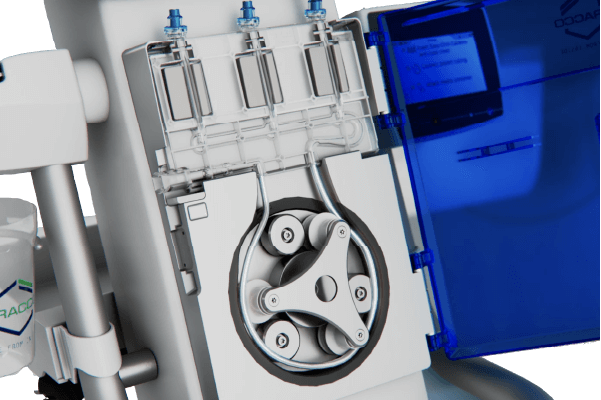
Roller Pump Technology
• Easy-Click-Cassette -flex: 24 hours of continuous injections
• Particle filtration

Ergonomic Design
• Small footprint with cable-free operation
• At least 18 hours of continuous runtime on lithium ion battery
MAXimize time savings when setting up for your next patient
Schedule your demo to see how the Max 3 system can deliver peak performance in your MR suite.
As a Bracco partner, you’re invited to sign up for an exclusive demo of the Bracco Max 3, next-generation syringeless MR injector system. The Bracco Max 3 can transform your MRI suite by decreasing your technologists’ time performing clinical tasks, while maximizing time caring for your patients.
Yes! Show me how to MAXimize my MRI suite!
Max 3
"*" indicates required fields
SEE THE OTHER PLAYERS
ON THE MR ROSTER.*
*Each of these products have been independently cleared or approved by FDA for use.
![]()
IMPORTANT SAFETY INFORMATION | ulricheasyINJECT Max 3™
The ulricheasyINJECT Max 3 injectors are not intended for the administration of contrast medium during high-pressure angiography or other applications that do not comply with the intended use.
The injector is not protected against the effects of defibrillation. Before a defibrillator is used, the patient must be disconnected from ulricheasyINJECT Max 3 injector.
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
ulricheasyINJECT Max 3™ (the Bracco-branded Max 3™, a Rapid Exchange and Syringeless MR Injector System) is distributed by Bracco Diagnostics Inc.
Indications for use
ulricheasyINJECT Max 3 (XD 10180) is a contrast media management system that is indicated for the controlled, automatic administration, on the venous side, of contrast media and saline (NaCl), to human subjects undergoing diagnostic examinations in magnetic resonance (MR) applications.
ulricheasyINJECT Max 3 (XD 10180) is specifically indicated for use in MRI procedures for the delivery of the following contrast media:
• Gadobutrol Injection in single-dose (SD) container or Imaging Bulk Package (IBP)
• Gadopiclenol Injection in SD container or IBP
• Gadobenate dimeglumine Injection in SD container
• Gadoterate meglumine Injection in SD container
Easy-Click-Cassette – flex Max 3 is used for a maximum time of twenty-four (24) hours or a maximum of 96 bottles of contrast media, whichever comes first.
Use time expiration per SD container is a maximum of four (4) hours, unless otherwise stated by the contrast media labeling.
Use time expiration per IBP or saline container is a maximum of twenty-four (24) hours, unless otherwise stated by the media labeling.
Spike for MRI disposable is for single-bottle use only and must be discarded with the media container. The Patient tubing must be discarded after each patient procedure.
ulricheasyINJECT Max 3 (XD 10180) is to be used only by and under quasi-continuous supervision of trained healthcare professionals in an appropriate licensed healthcare facility, in a room designated for radiological procedures that involve intravascular administration of contrast agent.
The ulricheasyINJECT Max 3 (XD 10180) is not intended for injection of contrast media (CM) for high-pressure angiography.
Contraindications
ulricheasyINJECT Max 3 injectors are not intended for the administration of contrast medium during high-pressure angiography or other applications that do not comply with the intended use.
The injector is not protected against the effects of defibrillation. Before a defibrillator is used, the patient must be disconnected from ulricheasyINJECT Max 3 injector.
Do not add any disposables (i.e. connector tubing or valves) to the ulricheasyINJECT Max 3 disposables or in conjunction with the patient tubing that are not provided by ulrich medical.
No valves or other connectors may be placed in-line between the patient tubing and the patient cannula. The disposables identified in this IFU are designed, manufactured, and tested for connection with cannulas for pressure injections.
Do not use ulricheasyINJECT Max 3 injectors with any other contrast media (other than those described in this IFU). Any other contrast media are inappropriate and should not be used.
Do not operate the injector and terminal, including any accessories, in potentially explosive atmospheres or in the vicinity of combustible materials (especially anesthetic drugs, detergents, and oxygen-enriched environments).
ulricheasyINJECT Max 3 is manufactured by ulrich GmbH & Co. KG.
ulrich medical is a registered trademark of ulrich GmbH & Co. KG.
ulricheasyINJECT Max 3 is a trademark of ulrich GmbH & Co. KG.
ulricheasyINJECT Max 3 is distributed as the Bracco-branded Max 3, a Rapid Exchange and Syringeless MR Injector System, by Bracco Diagnostics Inc.; 510 Carnegie Center, Suite 300, Princeton, NJ 08540 USA; Phone: (800) 631-5245; Fax: (609) 514-2424; Customer Service: 1-877-BRACCO 9 (1-877-272-2269); Scientific Information: 1-800-257-5181 (Option 2); Website: https://smartinject.com/max3/
All other trademarks and registered trademarks are the property of their respective owners.
References:
1. Data on File. Bracco Diagnostics Inc.; 2024




